1. The Natural greenhouse effect
1. The Natural greenhouse effect
1. The Natural greenhouse effect
Our planet, The Earth, is situated in its own greenhouse: the atmosphere. The greenhouse effect is natural, and life on Earth depends on it. Sunlight – energy from the sun – passes through the atmosphere and warms land and water surfaces. The amount of sunlight reaching the Earth is called insolation. Sunlight is perceived as colorless or as “white” light.
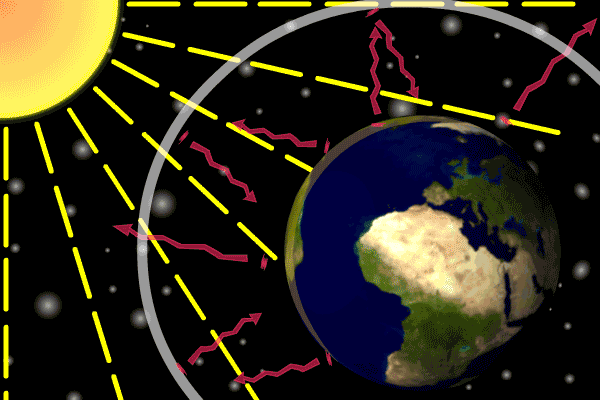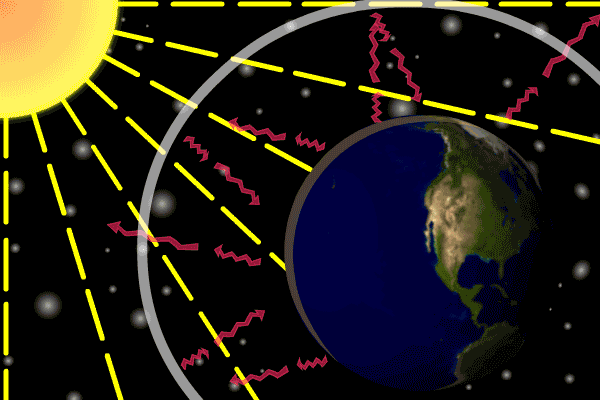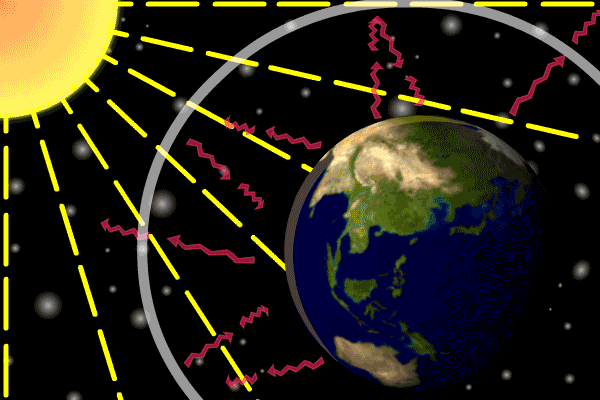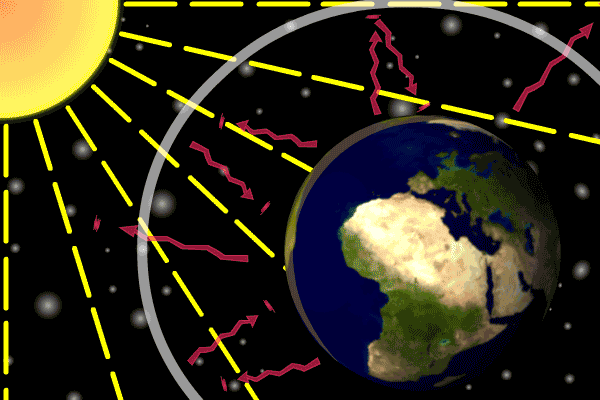
Figure 1. Sunlight heats the Earth’s. surface. The heat converts into infrared , diffuse radiation. Some of these infrared rays leave the atmosphere, some hit GHG gas molecules and warm the lower parts of the atmosphere. Animation: Yannick Urs Schillinger
Did you ever try sunbathing on cliffs on a nice summer’s day?
Even after sunset you can feel the heat from the rocks. The white sunlight has warmed the cliffs during the day. The warm cliffs emit rays of energy long after sunset. These rays of energy cannot be seen by humans unless we wear special glasses enabling us to see infrared. (The military uses this quite a lot) (Can we get a photo taken by infrared camera? ). The infrared rays of energy emitted to the air, go back to outer space or temporarily warms the air around us, keeping it warm even when the sun is not shining. In contrast to other planets in our solar system, the Earth thus has a natural temperature control system. Certain atmospheric gases are critical to this system and are known as greenhouse gases.
On average, about one third of the solar radiation that hits the earth is reflected back to space. Of the remainder, the atmosphere absorbs some, but the land and oceans absorb the most. The Earth’s surface becomes warm and as a result emits infrared radiation. Infrared light is heat energy.
Greenhouse gases are abbreviated GHG.
The greenhouse gases ‘trap’ the infrared radiation, thus warming the atmosphere.
Technically, when the infrared rays hit a GHG molecule, it starts vibrating.
When this molecule vibrates, it makes adjacent gas molecules of any kind to vibrate as well. Vibration is a form of energy that is converted to heat energy according to the physical laws of thermodynamics.
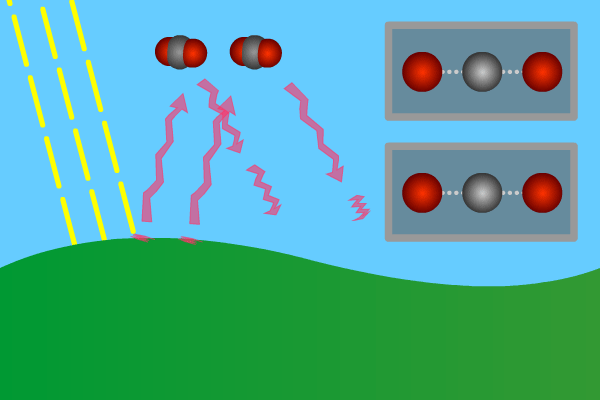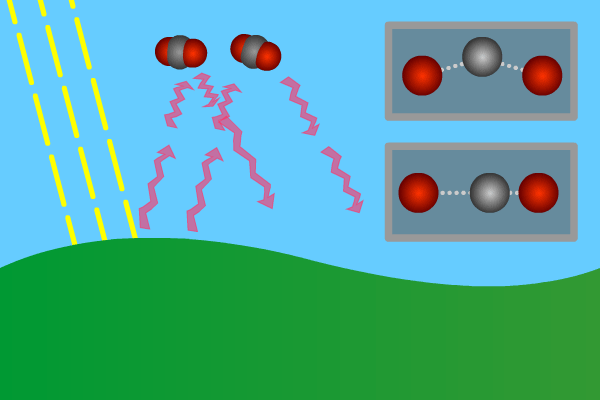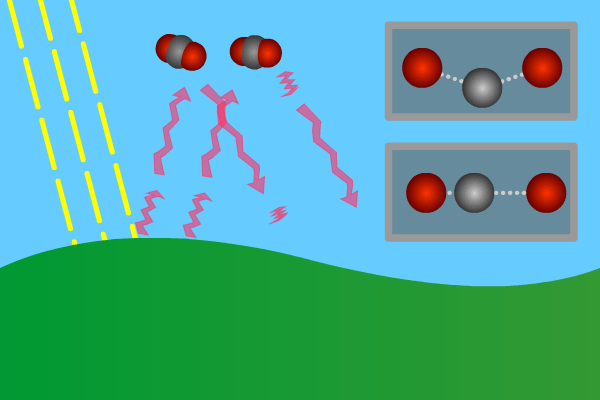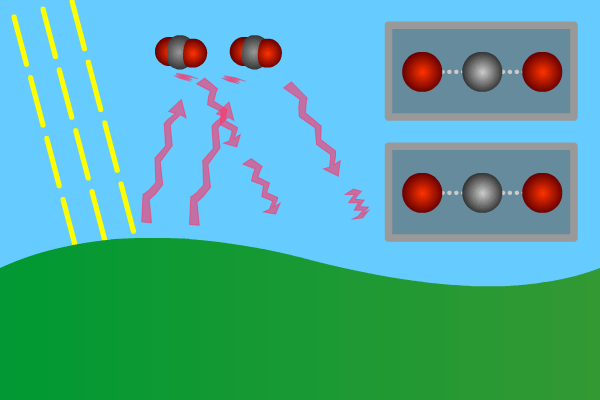
Figure 2. GHG molecules have three or more atoms. When hit by infrared long wave radiation, they vibrate, in turn hitting inert other molecules, making them also vibrate. Vibration is energy, in turn converted to heat, and warms the lower levels of the atmosphere (troposphere). Animation: Yannick Urs Schillinger
Close –up: Incoming Sun-rays warming the earth. Outgoing warm infrared hitting molecules of greenhouse gases: H2O, CO2, CH4, N2O making them vibrate.
The GHG molecule vibrations makes evenings and early nights feel warm after a sunny day. Without the GHG, the nights would immediately turn extremely cold at once the sunlight disappears. An increased greenhouse effect therefore can be observed as increasingly warmer nights.
Exercises:
- “Virtual laboratory”: Understanding the structure of the atmosphere is critical in understanding where and how global warming occurs. This visualization illustrates the major layers in the atmosphere and identifies a number of key characteristics and defining attributes of each layer.
- “Virtual laboratory”: Collisional heating of the amtosphere. Experiment with absorption of infrared radiation by CO2 in the troposphere and the collisional loss of this absorbed energy to surrounding N2 and O2 molecules. In this animation the user can sweep through a region of the IR spectrum and excite some of the vibrational modes of CO2. A simple (purely qualitative) thermometer illustrates the rise in temperature of the gas as collisional de-excitation occurs.
Read more:
BBC: Climate – (short films)
IPCC: What is the greenhouse effect?