1. The Natural greenhouse effect
| Site: | E-izglītība |
| Course: | Climate and sustainable development |
| Book: | 1. The Natural greenhouse effect |
| Printed by: | Vieslietotājs |
| Date: | Wednesday, 4 February 2026, 7:24 AM |
Description
1. The Natural greenhouse effect
1. The Natural greenhouse effect
Our planet, The Earth, is situated in its own greenhouse: the atmosphere. The greenhouse effect is natural, and life on Earth depends on it. Sunlight – energy from the sun – passes through the atmosphere and warms land and water surfaces. The amount of sunlight reaching the Earth is called insolation. Sunlight is perceived as colorless or as “white” light.
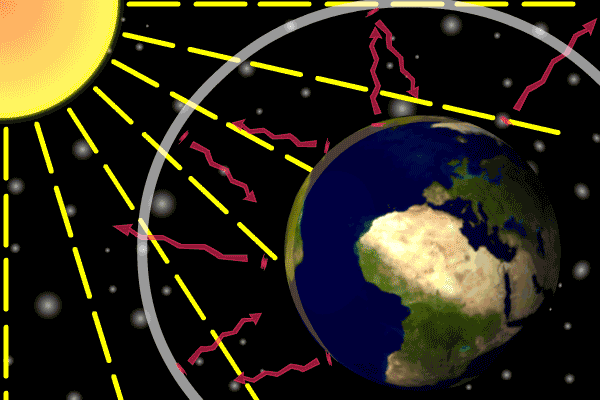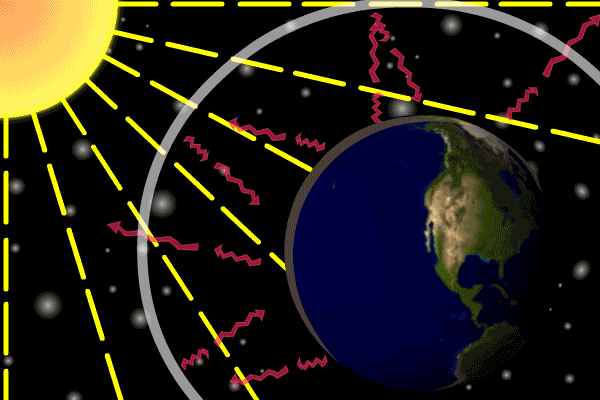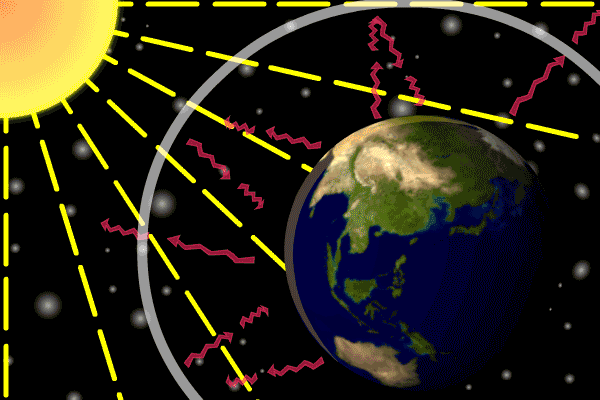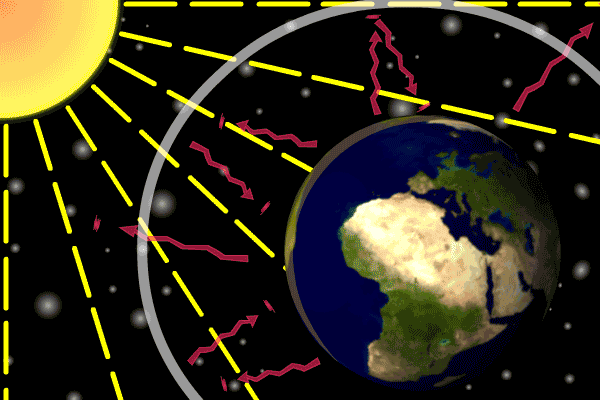
Figure 1. Sunlight heats the Earth’s. surface. The heat converts into infrared , diffuse radiation. Some of these infrared rays leave the atmosphere, some hit GHG gas molecules and warm the lower parts of the atmosphere. Animation: Yannick Urs Schillinger
Did you ever try sunbathing on cliffs on a nice summer’s day?
Even after sunset you can feel the heat from the rocks. The white sunlight has warmed the cliffs during the day. The warm cliffs emit rays of energy long after sunset. These rays of energy cannot be seen by humans unless we wear special glasses enabling us to see infrared. (The military uses this quite a lot) (Can we get a photo taken by infrared camera? ). The infrared rays of energy emitted to the air, go back to outer space or temporarily warms the air around us, keeping it warm even when the sun is not shining. In contrast to other planets in our solar system, the Earth thus has a natural temperature control system. Certain atmospheric gases are critical to this system and are known as greenhouse gases.
On average, about one third of the solar radiation that hits the earth is reflected back to space. Of the remainder, the atmosphere absorbs some, but the land and oceans absorb the most. The Earth’s surface becomes warm and as a result emits infrared radiation. Infrared light is heat energy.
Greenhouse gases are abbreviated GHG.
The greenhouse gases ‘trap’ the infrared radiation, thus warming the atmosphere.
Technically, when the infrared rays hit a GHG molecule, it starts vibrating.
When this molecule vibrates, it makes adjacent gas molecules of any kind to vibrate as well. Vibration is a form of energy that is converted to heat energy according to the physical laws of thermodynamics.
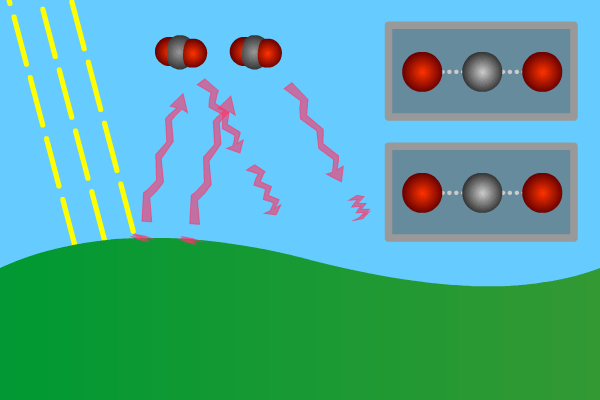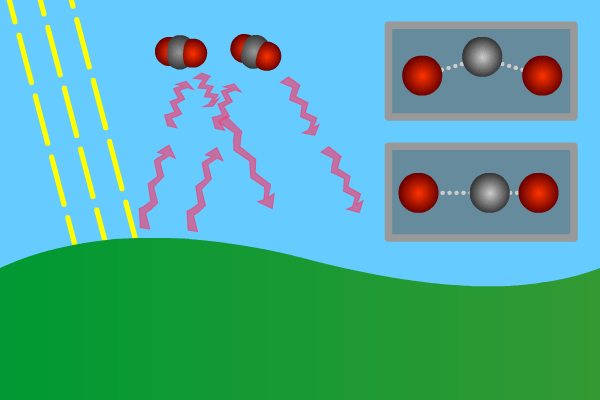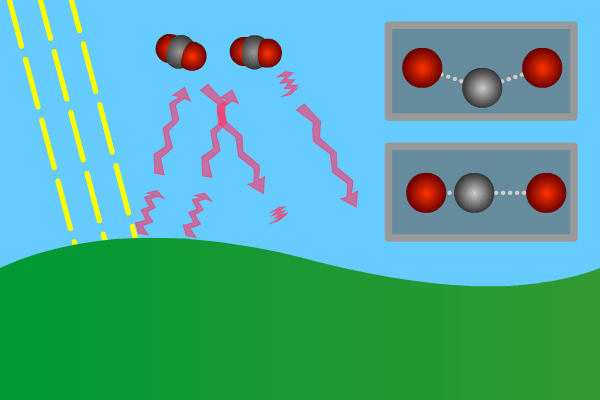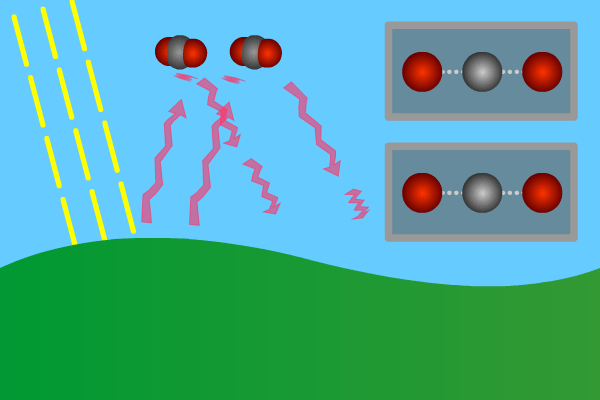
Figure 2. GHG molecules have three or more atoms. When hit by infrared long wave radiation, they vibrate, in turn hitting inert other molecules, making them also vibrate. Vibration is energy, in turn converted to heat, and warms the lower levels of the atmosphere (troposphere). Animation: Yannick Urs Schillinger
Close –up: Incoming Sun-rays warming the earth. Outgoing warm infrared hitting molecules of greenhouse gases: H2O, CO2, CH4, N2O making them vibrate.
The GHG molecule vibrations makes evenings and early nights feel warm after a sunny day. Without the GHG, the nights would immediately turn extremely cold at once the sunlight disappears. An increased greenhouse effect therefore can be observed as increasingly warmer nights.
Exercises:
- “Virtual laboratory”: Understanding the structure of the atmosphere is critical in understanding where and how global warming occurs. This visualization illustrates the major layers in the atmosphere and identifies a number of key characteristics and defining attributes of each layer.
- “Virtual laboratory”: Collisional heating of the amtosphere. Experiment with absorption of infrared radiation by CO2 in the troposphere and the collisional loss of this absorbed energy to surrounding N2 and O2 molecules. In this animation the user can sweep through a region of the IR spectrum and excite some of the vibrational modes of CO2. A simple (purely qualitative) thermometer illustrates the rise in temperature of the gas as collisional de-excitation occurs.
Read more:
BBC: Climate – (short films)
IPCC: What is the greenhouse effect?
1.1. Science of the natural Greenhouse Effect
Energy from the universe enters our planet, warming land, sea and air. The main source of energy is the sun. The planet warms during daytime, cools during nighttime. To keep the average temperature at the same level, the same amount of energy reaching the Earth, must leave. This is called the “energy balance ” of the Earth.
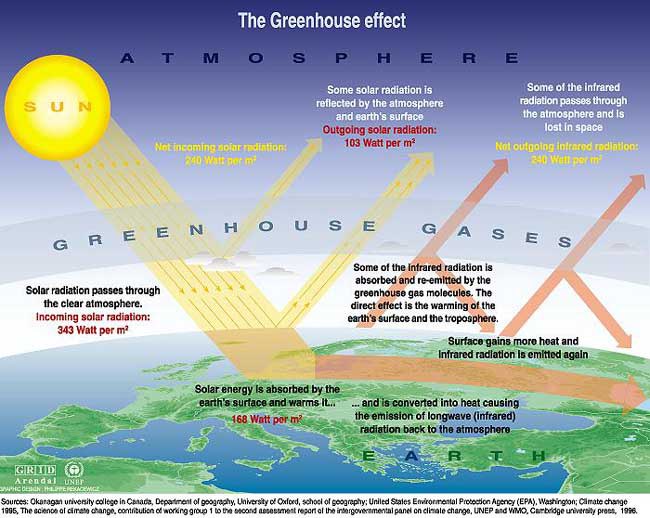
The amount of energy reaching the top of Earth’s atmosphere each second on a surface area of one square metre facing the Sun during daytime is about 1,370 Watts, and the amount of energy per square metre per second averaged over the entire planet is one-quarter of this: 343 Watts per m2.
About 30% of the sunlight, 103Watts per m2, reaching the top of the atmosphere is reflected back to space. Almost 70% of this reflectivity is due to clouds and particles in the atmosphere, the ‘aerosols’. Light-coloured areas of Earth’s surface such as snow, ice and deserts, reflect the remaining 30% of the sunlight.
Dramatic changes in aerosol reflectivity occur after major volcanic eruptions ejecting aerosols into the stratosphere. Aerosols in the troposphere are cleared by rain in a week or two. However, particles thrown above the troposphere roughly 10 km up in the atmosphere may remain floating in the air for months and years before falling into the troposphere. Volcanic eruptions can cause a drop in mean global surface temperature of about half a degree celsius that can last for several months.
Solar energy not reflected back to space is absorbed by the Earth’s surface and atmosphere. This amount is 240 Watts per square metre (W m–2). To balance the incoming energy, the Earth must radiate the same amount of energy back to space in the form of outgoing longwave, infrared radiation. Everything on Earth emits longwave radiation continuously. To emit 240 W m–2, the Earth’s surface would have to have a temperature of around –19°C; much colder than the conditions that actually exist at the Earth’s surface. The global mean surface temperature is about +14°C.
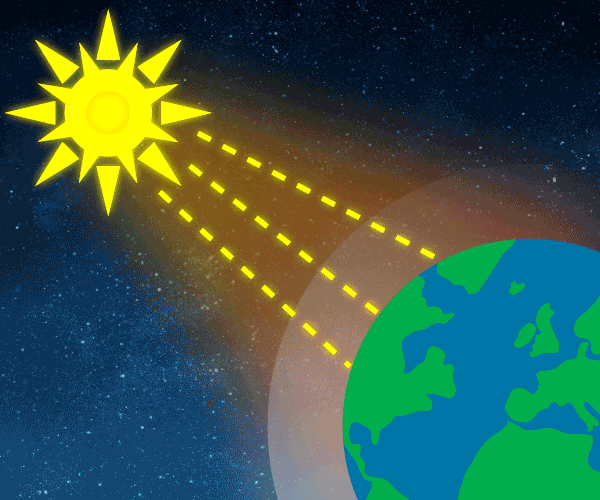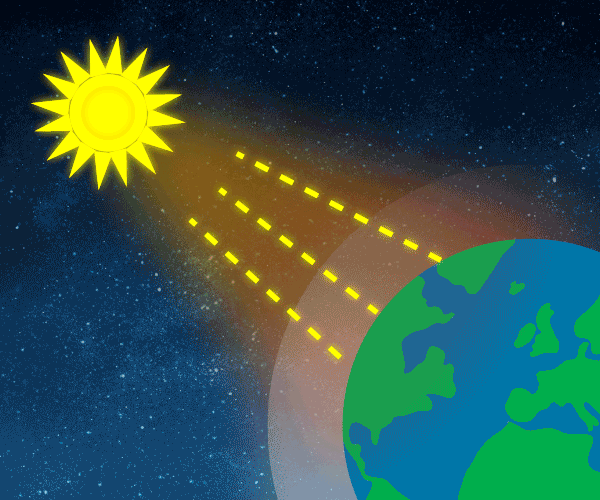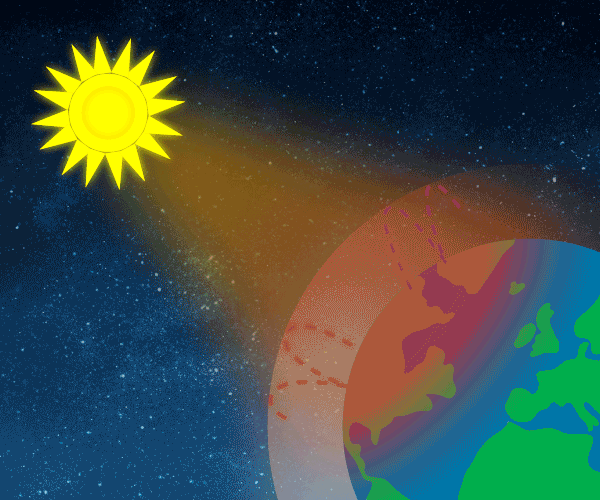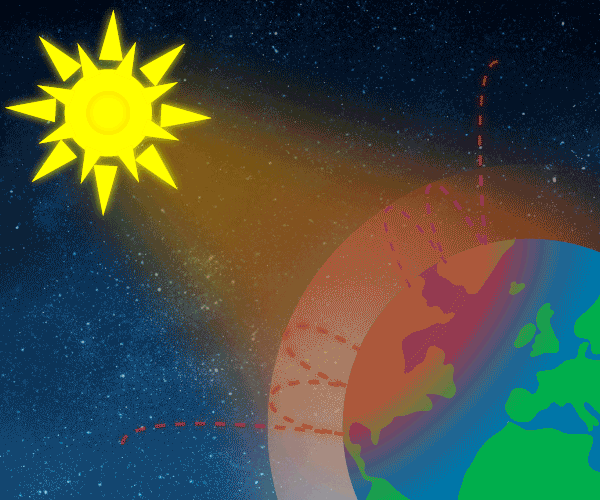
Graphic: Thomas Andersen, UIA.
The Earth’s surface is this warm due to the greenhouse gases, acting as a “blanket” for the longwave radiation coming from the surface. This blanketing is known as the natural greenhouse effect. The natural greenhouse effect thus makes a difference of almost 33°C.
The enhanced greenhouse effect has so far added almost one extra centigrade coming in addition to the 33°C existing naturemade centigrades.
- From IPCC: What Factors determine Earth’s Climate?
- NASA: The Earth’s energy budget
- Royal Society/climate change: An introduction to climate change in 60 seconds
- Pierrehumbert, R. (2011) Infrared radiation and planetary temperature
- Real Climate.org http://www.realclimate.org/
1.2. The Greenhouse Gases (GHG)
The greenhouse gases are components of the atmosphere that can ‘absorb’ and release infrared rays (heat energy). These gases have three or more atoms in their molecules. This in contrast to the gases making up more than 99% of the atmosphere: nitrogen, oxygen and argon having only two atoms in their molecules.
Water vapor (H2O)
The most important greenhouse gas is water vapor, which at times can approach 2% in parts of the atmosphere. However, when water vapor condenses into clouds, the white upside reflects sunlight back into space, which has a negative forcing effect.
Water vapor cannot initiate radiative forcing, it can only respond to radiative forcing through feedback mechanisms. Water vapor in the air is fairly constant, and human activities have little impact on this. Since humans have little or no direct impact on the amount of water in the air, and since it is not a forcing factor, water vapor is not included in negotiations on the reduction of emissions of greenhouse gases.
With increasing temperatures there will be increasing evaporation and increased amounts of water vapor in the air. In addition to enhancing the greenhouse effect it means that rainstorms can become more violent, as more water has to come down.
Carbon dioxide (CO2)
Carbon dioxide is the gas that almost all living creatures breathe out. Green vegetation absorbs the CO2 during daylight hours, assimilating it in the process called photosynthesis. At the same time the plants release the gas oxygen (O2 ), which animals and humans breathe in. Without oxygen we would suffocate.
The photosynthesis is the basis for all higher forms of life on Earth, as it in addition to oxygen produces glucose, a simple sugar that is the basis for more complex compounds building up the plants. Without plants, animals would no longer have food.
Increasing amounts of CO2 in the air may have a fertilizing effect on many plants if other essential requirements for photosynthesis are present. (Water, soil, minerals, right temperature etc).
Atmospheric CO2 has increased from a pre-industrial concentration of about 280 ppmv to about 400 ppmv year 2016.
(ppmv= parts per million by volume. This refers to the ratio of the number of greenhouse gas molecules to the total number of molecules of dry air. E.g.: 300 ppm = 300 molecules of GHG per million of dry air molecules.).
Atmospheric carbon dioxide (CO2 ) concentration was lingering around 280 ppmv for several thousand years until approximately 1750, the beginning of the Industrial Revolution. At that time large scale burning of fossil fuels started, while at the same time large-scale cutting of timber also took place.
NOAA: Carbon Dioxide Levels ‘Exploded’ in 2015, Highest Seen Since End of Ice Age
NASA (2016) Record annual increase of carbon dioxide observed at Mauna Loa for 2015
NASA: Graphic: The relentless rise of carbon dioxide
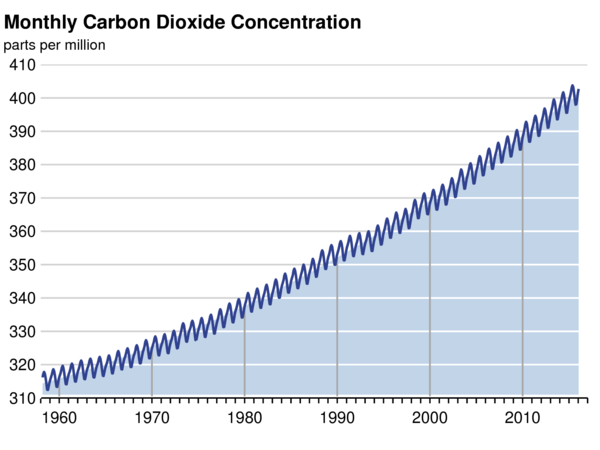
The Keeling curve – Mauna Loa observatory
The content of CO2 in the atmosphere has risen continuously since then, reaching 400 ppmv in 2015. This is an increase approaching 40%.
The present atmospheric CO2 concentration has not been exceeded during the past 420,000 years, and likely not during the past 20 million years. The rate of increase over the past century is unprecedented, at least during the past 20,000 years.
With “current energy consumption patterns the amount of carbon dioxide added to the atmosphere (as modified by absorption within natural sinks) is rising at a rate of about one half of one per cent per year” (WMO: Climate into the 21st century).
The present atmospheric CO2 increase is caused by anthropogenic emissions of CO2.
About three-quarters of these emissions are due to fossil fuel burning. Cement production and land use change is responsible for the rest of the emissions.
Methane (CH4)
This potent greenhouse gas is emitted from oil- and gas fields. Methane is released from rice paddies and from places where organic material rots without oxygen, like in marshes and big waste dumps.
Methane is also produced in the digestive system of animals, especially in ruminators like cows.
Large amounts of methane lie frozen in pockets in the Siberian permafrost areas and in frozen hydrates or clathrates on the sea floor. The Global Warming Potential (GWP) of methane is calculated to be 21 times that of CO2.
The increase of methane in the air is associated with the increase in human population and the connected increase in demand for energy, food, water, dwellings and waste dumps.
The content of methane in the atmosphere has increased about 2,5 times since 1750, from roughly 700 parts per billion by volume (ppbv) to 1803 ppbv in year 2011. This is an increase by 1060 ppb or 151%. The present concentration has not been exceeded during the past 420 000 years.
There are enormous methane deposits in ocean sediments and in permafrost areas in the Arctic. Life span of methane in the atmosphere is relatively short, only between 10-20 years, due to its reaction with oxygen.
Nitrous oxide (N2O)
This gas is emitted from artificial fertilizers in modern agriculture, from production of nitric acid and from combustion of fossil fuels. Nitrous oxide is responsible for approximately 4% of the anthropogenic greenhouse effect.
Nitrous oxide (N2O) in the atmosphere has increased from 270 ppbv in 1750 to 324 ppbv in 2011. This is an increase 20%. The increase continues.
Sources of N2O are agriculture, cattle, industry and several other natural sources. N2O is a strong greenhouse gas with a GWP of 310 times that of CO2 .The lifetime in the atmosphere is also quite long, more than 100 years.
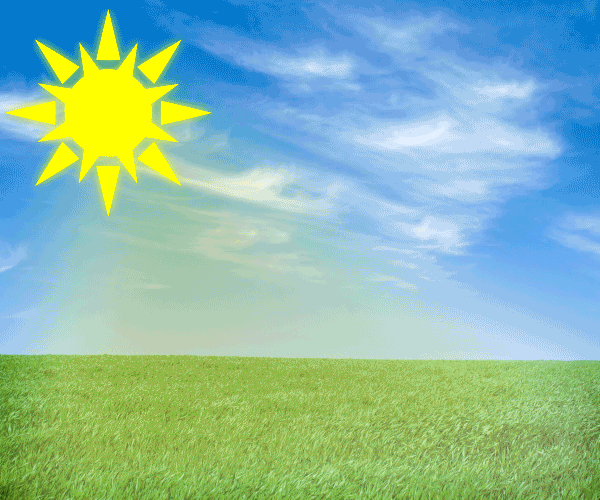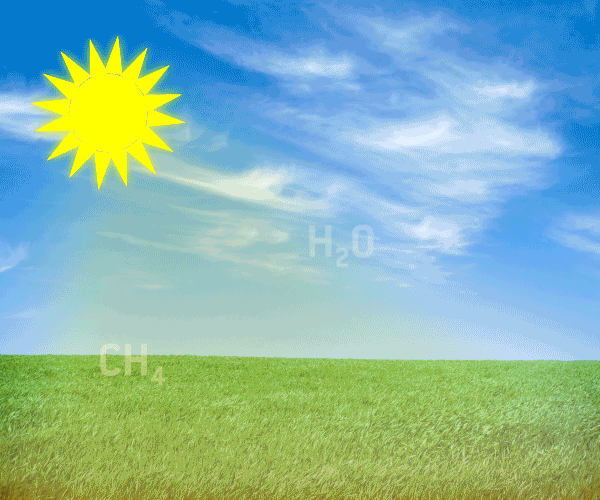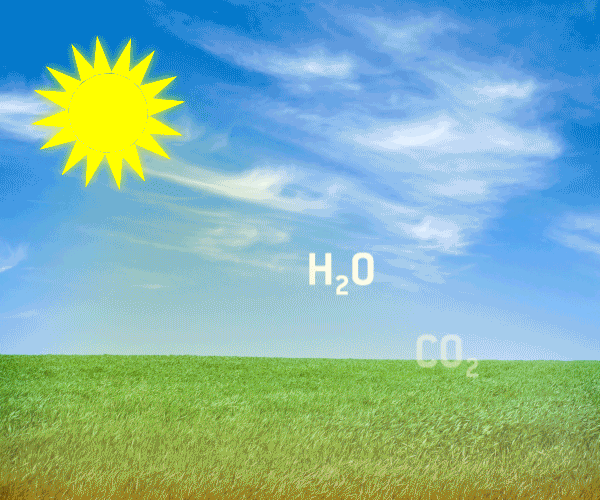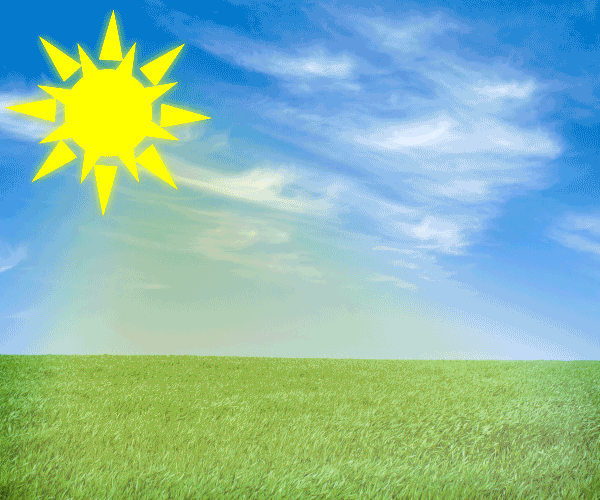
The natural greenhouse gases are continuously being produced in nature, and are parts of finely adjusted ecological systems occuring in natural cycles. Graphic animation: Thomas Andersen, UIA
Read more:
- Greenhouse gases
- Royal Society: CO2 is already in the atmosphere naturally, so why are emissions from human activity significant?
- Explaining how the water vapor greenhouse effect works
- Sources of Greenhouse Gas Emissions (EPA)
- The Atmosphere
- Methane
- Methane emssions (EPA)
- Nitrous oxide emissions (EPA)